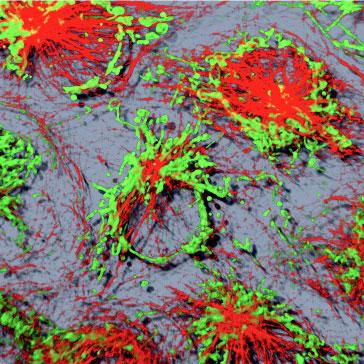
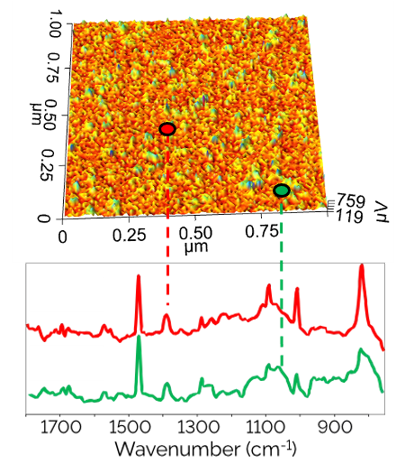
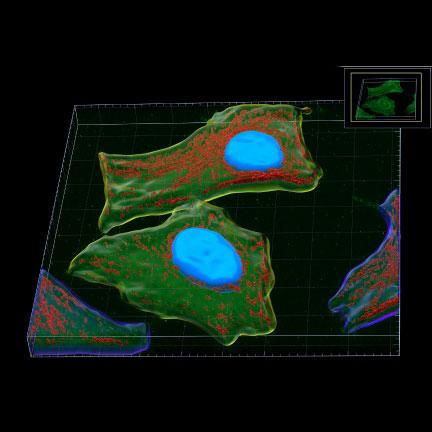
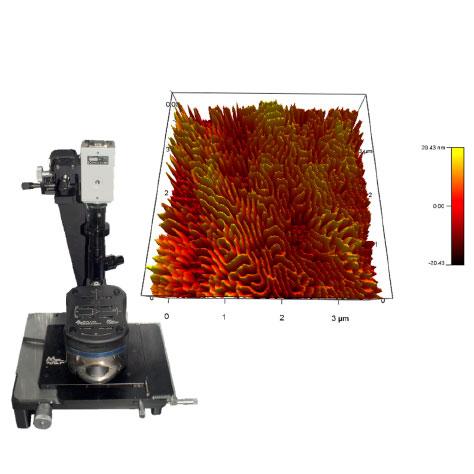
The Microscopy Core Facility provides researchers with the ability to visualize a variety of samples from single fibril and intracellular structures to very thick sections from organ tissues. This is accomplished using a variety of imaging technologies, including: Widefield, Light sheet (DLS), Confocal (LSM), Multi-photon, Total Internal Reflectance Fluorescence (TIRF), Super-Resolution Stimulated Emission Depletion (STED) microscopy, as well as equipment for cell and tissue laser dissection microscopy and live cell imaging and image analysis. The Core Facility also allows users to conduct biomechanical studies complemented by the Atomic Force Microscopy (AFM) including force spectroscopy experiments and imaging. The Microscopy Core Facility provides access to several workstations with software packages for image processing, analysis, and 3D image reconstruction. The Core Facility director offers instrument training, imaging and sample preparation assistance, as well as consultation for both on- and off-campus investigators of all levels of experience and from any discipline.
Contact Information:
Sergey Pryshchep, Ph.D.
Microscopy & Cellular Imaging Core Director
518-276-3456
pryshs@rpi.edu
- Wikipedia has evolved into a superb resource for microscopy enthusiasts.
- Olympus Microscopy Resource Center: a great website to learn more about basic and advanced microscopy theory and techniques.
- Nikon MicroscopyU: another great resource for tutorials and background.
- Zeiss’ History of Microscopy
- Excellent description of aldehyde-based fixatives by John Kiernan
- Molecular Probes (Invitrogen) fluorescence spectra viewer: an invaluable resource for designing experiments and choosing fluorescent labels.
- NIH’s ImageJ software: excellent open source image analysis software.
- University at Buffalo’s confocal listserv
- Histonet archives: for all your histology-related questions.
- Pierce (Thermo Fisher Scientific) maintains an excellent library of technical guides: the antibody technical handbook is outstanding
Access to the Microscopy Suite and Equipment
Access to all equipment must be approved by the core director. Approved users will have their ID cards activated to access the microscopy suite. Center users may request keys to the equipment rooms (limit one key per laboratory for each equipment room). All other users will need to request temporary access from the front desk.
Each individual user has a unique login name. Password sharing is prohibited. Doing so will lead to suspension of user’s access to the facility.
Training
All users must receive proper training before being permitted access to the equipment. Training is provided by the core director. The length of the training varies with the trainee’s prior experience on the equipment, but a typical training session will last about three hours. Minimum training time is one hour.
Scheduling
Advance reservation using the online sign-up is required. The billing is based on the length of occupation time, which is calculated based on the online sign-up records (minimum) and the equipment computer log records. Make sure to logout after each session.
Cancellation within 48 hours prior to the experiment will be subjected to a $20 cancellation fee.
Cleaning Up
After each session, users are required to remove oil from the objectives using the lens paper and the Sparkle solution (purple color) as demonstrated during the training. Do not rub the lens.
Users are responsible for keeping the equipment areas clean and for removing their data from the hard disk of the computer immediately after each session. Long term storage of large quantity of data on the equipment computer is prohibited.
Log Book
The logbook must be used to record the actual start and end time for each session and any problems encountered during the session.
Problems, Questions and Suggestions
Users are required to report to the core director any problems that occur on the equipment during the session immediately. If the core director is unavailable (e.g. after hours use), please e-mail the core director and leave a note for subsequent users if the problem affects the usability of the instrument. Questions and suggestions are always welcome.
Billing
All users are responsible for providing accurate and updated billing information. Users with multiple billing accounts should specify the account number to be charged upon sign-up. Both internal and external users will be billed on a monthly basis.
Core User Grants
Unfunded investigators who need to use the facility may apply for a core user grant. Eligibility will be determined by the Center Director, Dr. Deepak Vashishth.
Technology